Research Grants
Children with high risk cancers often have few options for treatment, and chemotherapies that bombard their systems with toxins continue to be in widespread use. We have provided research grants that help scientists take innovative approaches to finding safer treatments and offering kids better odds for survival. You can click on the links below to read more about the projects we have funded. We are not currently funding research grants and all donations will go to support the Damon Runyon - Jake Wetchler Award for Pediatric Innovation. We think Jake would be pleased with the punches we’ve landed for all the kids who today continue to fight cancer.

Development of Pharmacologic Agents to Target Leukemic Stem Cells.
Conducted by Dr. Tomasz Cierpicki at the University of Michigan. Funded in Partnership with The Leukemia & Lymphoma Society.
One of the leading theories as to why most AML treatments fail is that AML is driven by leukemic stem cells that self-renew and propagate the disease. Patients at first will seem to respond to treatment but then relapse because chemotherapy fails to kill the stem cells. Novel drugs that can kill leukemic stem cells are therefore urgently needed. Dr. Cierpicki’s research specifically targets these cells with the development of a potent inhibitor to block the activity of a complex called Ring1B-Bmi1 that leukemic stem cells need for self-renewal.
Dr. Cierpicki’s team has already developed a class of compounds that are able to bind to and inhibit Ring1B-Bmi1 activity –an accomplishment that no other labs have reported to date. In the current research, he proposes to develop potent Ring1B-Bmi1 inhibitors optimized with properties that are favorable for use as a drug and that will inhibit leukemia progression, serving as anti-cancer therapies with minimal side effects.
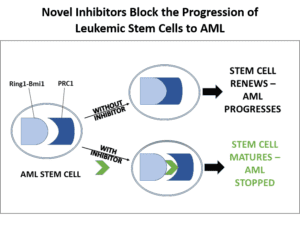
This research is innovative in two ways: its use of novel chemistry and its focus on an epigenetic target in AML stem cells. Unlike conventional chemotherapy, which seeks to kill tumor cells and has severe side effects from the unintentional killing of healthy cells, this epigenetic-based therapy is intended to promote cell differentiation, or the maturation of AML stem cells into normally functioning blood cells. Once mature, the cells would eventually get cleared from the body. The targeted results would be elimination of disease cells without toxic side effects.
The specific aims of the research are to (1) develop potent inhibitors of Ring1B-Bmi1 with optimized drug properties, (2) characterize the mechanism of action of Ring1B-Bmi1 inhibitors in leukemia cell lines, and (3) evaluate the therapeutic potential of targeting Ring1B-Bmi1 using mouse models and patient-derived AML cells.
The project team has a proven track record of taking novel medicinal chemistry from the lab and launching it on the path to patient treatments. They have developed a type of drug called menin-MLL inhibitors for treatment of certain leukemias and are collaborating with a biotech partner to test this treatment in preclinical studies. Similarly, for the current research, the long-term goal is to advance the inhibitors to preclinical and then clinical trials for acute leukemia patients via collaboration with a biotech company or the LLS Therapy Acceleration Program.

Dr. Stephen Chung
Characterization of CD99 as a Therapeutic Target in MDS and AML.
Conducted by Dr. Stephen Chung at the Memorial Sloan Kettering Cancer Center. Funded in Partnership with the American Society of Hematology.
Acute myeloid leukemia (AML) originates from leukemic stem cells, rare disease-driving cells that have the potential to both renew themselves and propagate other leukemia cells that make up the bulk of AML. Leukemic stem cells are largely resistant to conventional therapies, and they are believed to be the source of residual disease that causes patients to relapse even after they initially respond to therapy. Dr. Chung’s research is aimed at developing a new treatment that has the potential to stop relapse by targeting a specific protein on the surface of leukemic stem cells.
Dr. Chung has found that a protein called CD99 is expressed at greater levels on leukemic stem cells than on normal blood stem cells. It is similarly over-expressed on the stem cells that give rise to another related bone marrow disorder called the myelodysplastic syndromes (MDS.) In his preliminary lab work Dr. Chung has found that monoclonal antibodies targeting CD99 are directly toxic to primary AML and MDS cells, with minimal toxicity to normal blood-forming stem cells. This makes CD99 a promising target for low toxicity therapies that can kill AML and MDS stem cells while leaving normal stem cells alone.
Dr. Chung will now test his innovative approach to see if anti-CD99 monoclonal antibodies can eradicate human AML established in a mouse model by killing off both leukemic stem cells and bulk AML cells.
Dr. Chung will also explore the mechanism by which anti-CD99 drugs can be effective. CD99 regulates the activation of a group of enzymes called the “Src-family kinases,” and he is proposing that the monoclonal antibodies are effective because they cause rapid activation of these kinases, leading to lethal amounts of cellular stress. He will validate this mechanism as a potential novel therapeutic vulnerability in these diseases and identify other CD99-interacting partners or pathways that might also be targeted therapeutically. Finally, Dr. Chung will determine whether CD99 expression can be used as a marker of prognosis and/or minimal residual disease in AML.
The research findings are anticipated to lead to the development of new therapeutics for AML patients directed at CD99, the use of CD99 as a prognostic biomarker, and the identification/validation of pathways associated with CD99 that may also be targeted for therapeutic benefit.

Dr. Lihua Elizabeth Budde
Targeting CD123 using Chimeric Antigen Receptor T Cells for Treatment of Acute Myeloid Leukemia.
Conducted by Dr. Lihua Elizabeth Budde at the City of Hope Hospital. Funded in Partnership with the Damon Runyon Cancer Research Foundation.
This research will test an innovative new therapy in a clinical trial involving Acute Myeloid Leukemia (AML) patients for whom conventional chemotherapy has not worked. The researcher, Lihua Elizabeth Budde, MD, PhD, has developed a method to engineer the patient’s own T-cells (immune cells that fight disease) to specifically target and kill AML cells. The trial is a first-in-human study building on Dr. Budde’s extensive preclinical research on this method.
She is also developing ways to enhance the potency of the modified immune cells in killing leukemia cells by addressing “immune inhibitory checkpoints” that might otherwise dampen the T cells’ effectiveness. Further, she has plans to improve the safety of T cell therapy by designing a “conditional suicide switch” that provides better control and the potential to turn off the immune response when needed.
This therapeutic approach has the potential to change the treatment paradigm and may significantly improve the cure rate for patients with leukemia, with testing first in adults and ultimately in children. We are excited to be advancing the leading edge of cancer research and to be offering new hope to patients battling AML today.

Dr. Rafiq is working in the lab of Dr. Renier Brentjens, where the focus is on novel treatments that genetically engineer the patient's immune cells to recognize and kill cancer cells.
Engineered T cells against WT1 for Treatment of Acute Myeloid Leukemia.
Conducted by Dr. Sarwish Rafiq at the Sloan Kettering Institute for Cancer Research. Funded in Partnership with the Leukemia & Lymphoma Society.
One of the most promising frontiers in the fight against cancer is immunology, in which scientists engineer the body’s own immune system to seek out and kill cancer cells. Dr. Rafiq plans to take T cells, which are the cells that normally identify and destroy infection but are unable to recognize cancer, and engineer them to recognize and kill acute myeloid leukemia (AML) cells.
Dr. Rafiq will design T cells to hone in on a protein called Wilms-Tumor 1 (WT1), which is expressed on the surface of AML cells. Dr. Rafiq plans to further enhance the T cell’s potency by having them release a protein called IL-12. She will test the treatment against AML patient samples and mouse models with the ultimate goal of bringing a highly targeted, low-toxicity treatment to the clinic for AML patients.
This research builds on experimental techniques developed by another team of scientists that were successful at saving the life of a seven year old girl with relapsed acute lymphoblastic leukemia, as reported in the New York Times earlier this year.

Dr. Palchaudhuri in the lab
Controlling Cell Differentiation through Modular Assembly of Cell-permeable Transcription Regulating Proteins.
Conducted by Dr. Rahul Palchaudhuri at Harvard University. Funded in Partnership with the Life Sciences Research Foundation.
Healthy blood cells follow an established life cycle, starting as stem cells, developing into blood cell progenitors, and then differentiating into specific types of cells. Properly differentiated cells mature and eventually die. In many cases of AML, however, the cells are genetically damaged so they never differentiate and mature, and instead proliferate out of control.
Dr. Palchaudhuri’s aim is to re-program the cellular processes of leukemic cells so that normal cell differentiation can once again occur, enabling blood cells to mature and die. He is targeting the abnormal activity of two “homeodomain proteins” which block the differentiation of myeloid cells, and plans to engineer “cell-permeable homeodomain fusion proteins” (HFPs) to stop the instructions that block differentiation. He will also use a novel technique called “hydrocarbon peptide stapling” to control the shape of the HFPs and enable them to enter the cell.
Dr. Palchaudhuri will first develop the HFPs and determine if they properly bind to their target, then test whether the HFPs induce AML cell differentiation in culture and in a mouse model. If successful, the approach would offer the promise of differentiation-inducing therapy for approximately 70% of AML patients. By overcoming current limitations in DNA-targeting technologies, it may be readily extended to other diseases as well.

Dr. Cihangir Duy
Targeting BCL6 in Acute Myeloid Leukemia (AML) Stem Cells.
Conducted by Dr. Cihangir Duy at the Weill Cornell Medical College. Funded in Partnership with the Leukemia & Lymphoma Society.
While conventional chemotherapy kills fast-growing AML cells, it often fails to destroy a group of stem cells and other "survivor" cells. As a result, the patient may seem to be responding but the cancer inevitably returns. The survivor cells are able to outwit existing treatments through a process in which they go dormant and therefore "under the radar" of chemotherapy, then resume activity once the threat is gone.
Dr. Duy is taking a three-pronged approach to target stem and other survivor cells. He has found that cells under stress reprogram their cellular functions through specific “epigenetic” changes (that is, changes that alter the instructions the genes would otherwise provide.) He also found a protein called “ATR” to be a main player in the cell’s stress response. By targeting two key epigenetic regulators, Dr. Duy has been able to successfully re-sensitize some drug-resistant survivor cells to chemotherapy. In addition, by inhibiting ATR in combination with chemotherapy, he has been able to compromise AML cell ”dormancy”, resulting in death of all survivor cells. His upcoming work will address the third prong of attack, determining how the protein BCL6 protects cancer cells and whether the inhibitor RI-BPI will disrupt this function.
Dr. Anne Bothmer
Identification and characterization of novel ribosome-associated proteins and their role in the development of blood cancers.
Conducted by Dr. Anne Bothmer at the Beth Israel Deaconness Medical Center, Harvard Medical School. Funded in Partnership with the Damon Runyon Cancer Research Foundation.
Dr. Anne Bothmer will peer into the “black box” of cellular machinery to determine how protein production in cancerous cells differs from that in healthy cells, and how those differences may lead to diseases such as acute myeloid leukemia (AML.) She is investigating a major pathway that affects more than a third of AML patients: the development of cancer from a mutation in the gene NPM1 and the resulting abnormal functioning of a crucial regulator called NPM.
Dr. Anne Bothmer will be working with Dr. Pier Paolo Pandolfi at Beth Israel Deconness Medical Center, using a novel mass spectrometry method to analyze differences between normal and cancerous cells. She will also be working in collaboration with Dr. Robert Darnell at Rockerfeller Unversity using techniques of high-throughput sequencing of RNA to understand how the absence of NPM alters the landscape around cellular machinery and can lead to AML and other blood cancers
Dr. Bothmer’s theory is that when NPM's functioning is impaired, cellular complexes called "ribosomes" that coordinate the fabrication of proteins are altered and as a result, prevented from playing their intended role in the production of blood cells. As shown in the diagram below, the questions of how ribosomes are altered and the role they play in blood cancers remains a mystery. With the results of Dr. Bothmer’s research, we should be better able to understand the mechanism that leads from gene mutation to disease, and reveal novel targets for therapy that would not otherwise be evident.

Antibody Conjugates for Targeted AML Therapy
Conducted by Dr. Hua Lu at the Scripps Research Institute. Funded in Partnership with the Damon Runyon Cancer Research Foundation.
Dr. Hua Lu is working on the development of a new “antibody conjugate” technology to more precisely attack acute myeloid leukemia (AML) cells while leaving healthy cells intact. If successful, the technology will be a major breakthrough both for improving survival and reducing side effects in AML and potentially other cancers as well.
Dr. Lu is a rising star in the field of chemistry and brings to his work a passion for finding better treatments for cancer and a novel idea for synthesizing ADCs. He is working in the lab of Dr. Peter Schultz (http://schultz.scripps.edu/main.php), a seasoned researcher and pioneer of breakthrough drug development techniques.
Hear Dr. Lu speak about his research and his motivation to fight back against cancer.
Dr. Lu is developing a unique technology that links molecules in very precise ways to an antibody, forming “antibody conjugates” that can recognize and kill cancer cells while leaving healthy cells unharmed. Unlike conventional chemotherapy, antibody conjugates function like a biological GPS system to zero in on just the cancer cells, and when designed with precision, they have the potential for greatly reduced toxicity and improved survival. In addition to tracking down the cancer cells, Dr. Lu’s antibody conjugates work by bringing cells from the body’s own immune system to the disease site and activating them to kill the cancer.
Dr. Lu has successfully synthesized two “biFab” antibody conjugates intended to locate and bind to acute myeloid leukemia (AML) cells while at the same time binding and activating the patient’s own killer T-cells to destroy them. His tests on the first antibody conjugate showed a strong ability to bind to the AML cells. In further experiments, he examined cancer killing ability using an AML cell line and found that the antibody conjugate showed potent cancer killing as early as 24 hours post-incubation. The second antibody conjugate showed similar potency in killing AML cells.
Dr. Lu is now developing an animal model for testing the efficacy of the antibody conjugates and will perform a tumor reduction study using both of them. He will also test the two antibody conjugates in AML patient samples. Dr. Lu has made enormous progress and we are very excited about the promise his results hold for effective, low toxicity treatments for children with AML.
Why the research is so important
One of the problems with conventional chemotherapy is that it kills cells indiscriminately, attacking not just cancer cells but healthy cells as well. By some estimates, only 1% of chemotherapy actually finds its target, the rest causing toxic side effects. Children in particular can suffer from serious and lifelong complications, including reduced heart function, respiratory damage, and diminished cognitive ability. Some treatments can even trigger a second cancer: the combination of chemotherapy and radiation used to treat Jake’s Hodgkin’s lymphoma was likely responsible for causing the AML which took his life.
Antibody conjugates are intended to overcome this problem by delivering drug directly and solely to disease cells, using an antibody as a biological “GPS system.” The goal is to more effectively attack the disease while leaving healthy cells unharmed.
However, initial attempts at developing antibody conjugates, particularly for AML, have fallen short because scientists have been unable to fully control how molecules attach to the antibody. Haphazard positioning can change the shape of the antibody or cover up the sites it needs to locate and bind to the cancer cells. “Blinded” in this way, the conjugate fails to fully deliver its payload to its target and instead can cause harm elsewhere in the body.
Dr. Lu’s innovative technology is intended to create chemical “handles” to hold molecules in place, allowing the antibody to accurately find the cancer cells.
If successful, the technology could be used not just for AML but for the treatment of a variety of cancers. The breakthrough would be especially important for children, whose still-developing systems would be spared chemo’s harmful effects, and who would be given a better chance at living full and healthy lives.
With this as our initial project, we’ve landed a solid first punch in the fight against pediatric cancer, for Jake and all the kids who today need better treatments and cures.